By Danny J. Soares MD , Stephanie D. Hynes NP-C , Christina H. Yi MD, Sabrina Shah-Desai MS, FRCS, Steven C. Irving MD
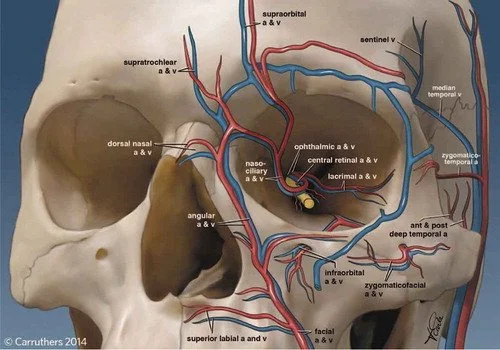
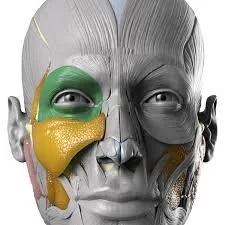
Vascular emergencies from cosmetic filler–induced vascular occlusion represent an iatrogenic etiology that poses a threat to patients, with sequelae that range from disfiguring skin necrosis to blindness and stroke. As cosmetic fillers continue to grow in popularity, the importance of early identification, triaging, and management of these rare but potentially disabling injuries has motivated efforts to educate the public and professional audiences. In this practice review article, we outline components of acute care pertaining to these injuries based on evolving practice guidelines and best evidence recommendations.
Read More